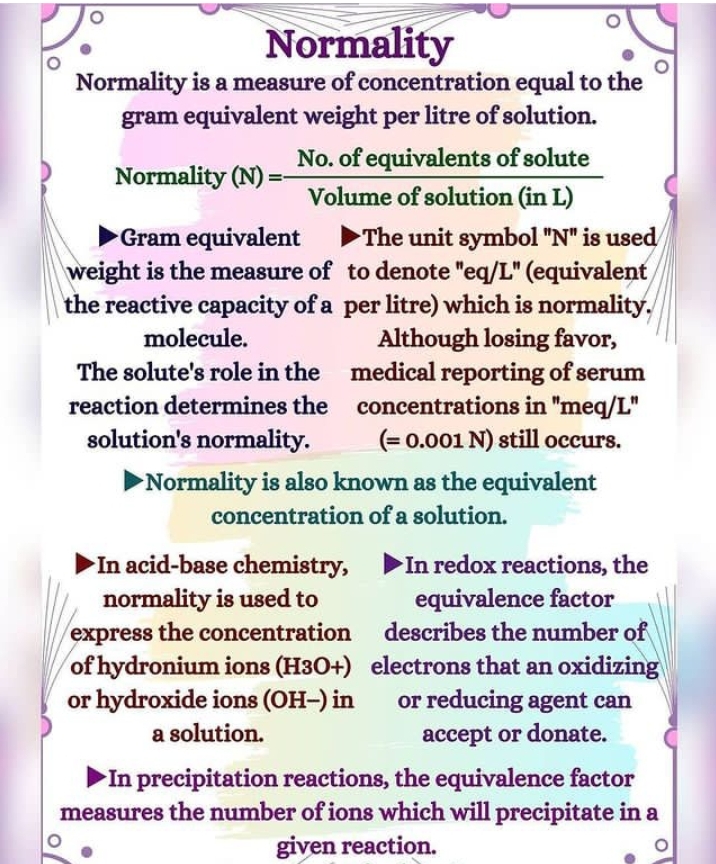
(Normality) N :
A solution formed by dissolving 1 gram of a substance in 1 liter of solution is called a 1 normal solution or the normality of the solution is 1.
Normality = gram liter-1 equivalent
= Weight of solute in grams Equivalent/ weight X volume of solution in liters
Equivalent weight of acid = Molecular mass of acid (grammole 1) /Basicity of acid
Basic equivalent weight = Basic atomic mass (grammol-1)/Acidity of Bayes
Example 1.5 : 73 g of hydrochloric acid was dissolved in water to make a solution of 500 ml. Calculate the normality of this solution.
Solution : Molecular mass of HCI = 36.5 grammol 1
Atomic force and equivalent force are same.
Equivalent mass of HCI = 36.5 g equivalent -1
Volume of solution 500 ml = 0.5 liter
Weight of soluble HCl = 73 g
Symmetry = weight of solute in grams x volume of solution in liters
Normality = 73 grams 36.5 grams x 0.5 liters = 4 N
Chemistry with Confidence preface In the intricate world of chemistry, where motes dance, responses unfold, and rudiments bond, chancing normalcy amidst complexity can be both grueling and satisfying.
learning chemistry isn’t just about learning equations or balancing responses; it’s about understanding the underpinning principles, embracing the complications, and chancing comfort in the normalcy that governs this fascinating discipline.
Understanding Normalcy in Chemistry Normalcy in chemistry transcends the notion of average or typical; it encompasses the foundational generalities, principles, and actions that govern chemical responses and relations. Let’s explore how normalcy manifests in colorful aspects of learning chemistry

** Fundamental Principles **
At the core of chemistry lies a set of abecedarian principles that govern the geste of matter and energy. From the laws of thermodynamics to the principles of stoichiometry, these foundational generalities give a frame for understanding and prognosticating chemical marvels.
Embracing these principles is essential for learning chemistry and unraveling the mystifications of the molecular world
** Precision and Accuracy **
In chemistry, perfection and delicacy are consummate. Precision refers to the reproducibility of measures, while delicacy refers to the closeness of a dimension to the true value.
Achieving both perfection and delicacy requires scrupulous attention to detail, proper estimation of instruments, and adherence to experimental protocols.
By seeking for perfection and delicacy, druggists insure dependable and reproducible results, laying the root for farther disquisition and discovery.
** Experimental ways **
Experimental ways play a pivotal part in chemistry, allowing scientists to observe, manipulate, and dissect matter at the molecular position. From titrations to spectroscopy, these ways give precious perceptivity into the parcels and geste of chemical substances.
learning experimental ways requires practice, tolerance, and a keen understanding of the underpinning principles. By honing their experimental chops, druggists gain confidence in their capability to design and execute trials, paving the way for new discoveries and improvements.
** Problem- working Chops **
Chemistry is innately problem working. Whether it’s balancing chemical equations, prognosticating response issues, or expounding molecular structures, druggists are constantly faced with challenges that bear creative thinking and logical logic.
Developing strong problem- working chops is essential for learning chemistry, as it enables druggists to attack complex problems with confidence and imagination.
By approaching problems totally, breaking them down into manageable way, and using their knowledge of chemistry, scholars and experimenters likewise can navigate the complications of the discipline with ease. Embracing Normalcy in the literacy Process learning chemistry isn’t a direct trip; it’s a dynamic process of disquisition, discovery, and growth.
Embracing normalcy in the literacy process involves admitting the ups and campo, the successes and failures, and chancing comfort in the eclipse and inflow of scientific inquiry.
Then are some strategies for embracing normality in the trip of learning chemistry
** continuity and Adaptability **
Chemistry can be grueling , and lapses are ineluctable. Whether it’s a failed trial, a miss new conception, or a delicate problem, it’s essential to approach obstacles with adaptability and determination. By embracing failure as an occasion for literacy and growth, druggists can overcome challenges, upgrade their chops, and eventually achieve success in their trials.
** Collaboration and Community **
Chemistry is a cooperative bid, and literacy is frequently most fruitful when participated with others. Whether through study groups, exploration brigades, or online communities, engaging with peers and instructors can give precious support, guidance, and perspective.
By fostering a sense of community and collaboration, druggists can navigate the complications of the discipline together, drawing strength from collaborative knowledge and experience.
** Curiosity and Wonder **
At its core, chemistry is driven by curiosity and wonder – the desire to understand the world around us and uncover its mystifications. Cultivating a sense of curiosity and wonder is essential for maintaining enthusiasm and passion for the discipline. Whether it’s marveling at the beauty of a demitasse chassis or pondering the complications of a chemical response, embracing the admiration- inspiring aspects of chemistry can reignite the spark of curiosity and energy continued disquisition and discovery.
Conclusion
In the pursuit of learning chemistry, chancing normalcy amidst complexity is both a challenge and a honor. By embracing the abecedarian principles, perfection and delicacy, experimental ways, and problem- working chops that define the discipline, druggists can navigate the complications of the molecular world with confidence and grace. also, by embracing normalcy in the literacy process – admitting the ups and campo, fostering adaptability and collaboration, and cultivating curiosity and wonder – druggists can embark on a trip of disquisition, discovery, and growth that transcends the boundaries of the laboratory and enriches their lives in profound ways.