Introduction:- Atomic theory
Today will about this Atom in Mastering Chemistry your overall health and vulnerability to complaint depends on complex relations between your inheritable makeup and environmental exposures.
the consequences of which are delicate to prognosticate. Beforehand discovery of biomarkers, which uphold an organism’s complaint or physiological state, may allow opinion and treatment after a condition has come reversible or reversible.
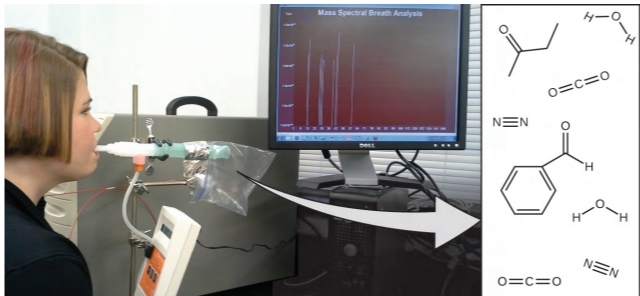
Recent studies have shown that your MP breath may contain motes that can lead to pathological conditions ranging from asthma or asthma to lung cancer. Scientists are working to develop a biomarker” point” that can be used to diagnose specific DG grounded on the number and identity of specific motes in a case’s MPs.
This thing is basically the conception of molecular identity, which is determined by the number and type of tittles and how they’re clicked together. This chapter will describe some introductory chemical principles that cover matters central to the conception of molecular recognition.
Initial Concepts of AtomicTheory
State the presuppositions of Dalton’s infinitesimal proposition. Use presuppositions of Dalton’s infinitesimal proposition to explain the laws of defi- nite and multiple proportions. The language used in chemistry is seen and heard in numerous disciplines, ranging from drug to engineering to forensics to art.
The language of chemistry includes its own vocabulary as well as its own form of shorthand. Chemical symbols are used to represent tittles and rudiments. Chemical formulas depict motes as well as the composition of presentations.
Chemical equations give information about the quality and volume of the changes associated with chemical responses. The generalities of this foundation include the infinitesimal proposition, the composition and mass of an snippet. the variability of the composition of isotopes, information, chemical bonds in ionic and covalent composites, the types of chemical responses, and the picking of compounds. We’ll also introduce one of the most important tools for organ- izing chemical knowledge the periodic table.
Nineteenth-century atomic theories
The foremost recorded discussion of the introductory structure of matter comes from ancient Greek proponents, the scientists of their day. In the fifth century BC, Leucippus and Democritus argued that each matter was composed of small, finite particles .they called atomos, a term deduced from the Greek word for “ inseparable. ”
They allowed of tittles as moving patches that differed in shape and size, and which could join together. latterly, Aris- totle and others came tothe conclusion that matter comported of colorful combinations of the four “ rudiments ” fire, earth, air, and water and could be infinitely divided. Interestingly, these proponents allowed about tittles and “ rudiments ” as philosophical concepts. but supposedly noway considered performing trials to test their ideas.
The Aristotelian view of the composition of matter held sway for over two thousand times, until English school teacher John Dalton helped to revise chemistry with his thesis. that the geste of matter could be explainedusing an infinitesimal proposition.
First published in 1807, numerous of Dalton’s suppositions about the bitsy features of matter are still valid in ultramodern infinitesimal proposition. Then are the presuppositions of Dalton’s infinitesimal proposition.
1. Matter is composed of exceedingly small patches called tittles. An snippet is the lowest unit of an element that can share in a chemical change.

2. An element consists of only one type of snippet, which has a mass that is characteristic of the element and is the same for all tittles of that element.
3. tittles of one element differ in parcels from tittles of all other rudiments.
conclution
To sum up, studying atoms and molecules is essential to comprehending the basic components of matter. We can progress several scientific areas and technologies by learning more about their structures, characteristics, and interactions. Our perception of the world around us is shaped by the research of atoms and molecules, whether it is for the purpose of solving cosmic riddles or creating novel materials.
https://en.m.wikipedia.org/wiki/Atom

5 thoughts on “Atom”